Public Alerts
N-Propionitrile chlorphine belongs to an emergent subclass of novel synthetic opioids often referred to as “orphine analogues” (or more simply “orphines”) and bears structural similarity to other benzimidazolones (e.g., brorphine, chlorphine). These drugs have ties to pharmaceutical drug discovery conducted in the 1960s and 1970s, beginning with substances like bezitramide and R-6890 (now referred to as “spirochlorphine”). The orphine analogues first emerged in recreational drug markets in 2020 with the proliferation of brorphine (a drug first synthesized and published on in 2018). This novel opioid subclass continues to diversify, with at least six analogues confirmed in recent years. N-Propionitrile chlorphine was first detected at the Center for Forensic Science Research and Education (CFSRE) in mid-2024. In vitro pharmacology data show this drug to be approximately 10x more potent than fentanyl [Vandeputte & Stove, personal communication]. The positivity of N-propionitrile chlorphine, specifically in fatal drug overdoses, has increased since mid-2025. In July 2025, the Chinese government placed nitazene analogues under generic control. Since this announcement, overall positivity for nitazene analogues has declined as overall positivity for orphine analogues has increased, led in large part by N-propionitrile chlorphine.
N-Propionitrile chlorphine has been identified in 25 blood specimens from fatal overdoses tested at the CFSRE, the vast majority submitted in late-2025 and early-2026. In addition, N-propionitrile chlorphine has been tentatively identified in more than 100 toxicology cases at NMS Labs. Toxicology specimens originated from nine states across the United States, as well as three provinces in Canada. N-Propionitrile chlorphine was detected as the sole opioid in 11 of 25 cases, and alongside other opioids (e.g., fentanyl, oxycodone) and traditional stimulants (e.g., methamphetamine, cocaine). Co-detection with NPS was common (e.g., novel benzodiazepines [phenazolam], other orphine analogues [spirochlorphine], nitazene analogues, and carfentanil).
Following the recent core structure scheduling by China of the nitazenes (benzimidazoles), markets have seen a decline in these potent opioids in late 2025, and their replacement by a new class of synthetic opioids, the benzimidazol-2-ones, also known as the “Orphines”. This alert describes the emergence of these potent opioids, and spotlights their detection in US and international drug markets.
---Brorphine was the first highly potent synthetic opioid of the benzimidazol-2-one (“orphine”) class, detected in European drug markets around 2019, and in the US in 2020. It likely originated from clandestine synthesis in China, emerging as a fentanyl analog-replacement or opioid adulterant with potency similar to or slightly less than fentanyl.
---Following initial alerts, brorphine was rapidly scheduled or emergency-controlled in multiple jurisdictions (e.g., EU, UK, US, Canada). This regulatory response appears to have accelerated structural diversification, with multiple halogenated and cyclized analogs appearing about four years after brorphine controls were put in place.
---As analytical reference standards have become more widely available, orphine analogs detected now include Chlorphine, N-Propionitrile Chlorphine (Cychlorphine), 5,6-Dichloro Desmethylchlorphine (SR-17018), Spirochlorphine (R-6890), Spirobrorphine and 5,6-Dicholoro Brorphine (SR-14968), various members of which have been reported in the UK, Europe, US, and Canada.
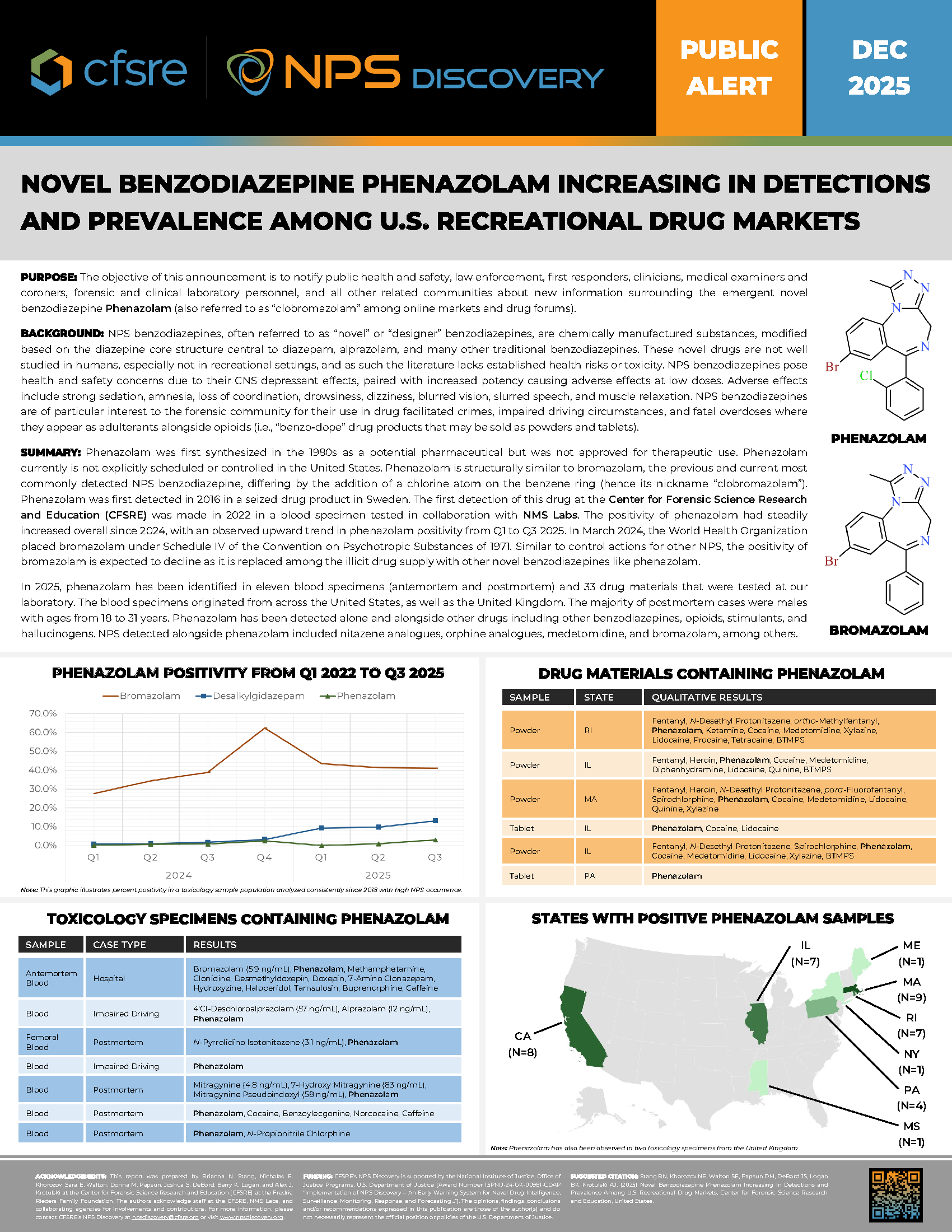
Phenazolam was first synthesized in the 1980s as a potential pharmaceutical but was not approved for therapeutic use. Phenazolam currently is not explicitly scheduled or controlled in the United States. Phenazolam is structurally similar to bromazolam, the previous and current most commonly detected NPS benzodiazepine, differing by the addition of a chlorine atom on the benzene ring (hence its nickname “clobromazolam”). Phenazolam was first detected in 2016 in a seized drug product in Sweden. The first detection of this drug at the Center for Forensic Science Research and Education (CFSRE) was made in 2022 in a blood specimen tested in collaboration with NMS Labs. The positivity of phenazolam had steadily increased overall since 2024, with an observed upward trend in phenazolam positivity from Q1 to Q3 2025. In March 2024, the World Health Organization placed bromazolam under Schedule IV of the Convention on Psychotropic Substances of 1971. Similar to control actions for other NPS, the positivity of bromazolam is expected to decline as it is replaced among the illicit drug supply with other novel benzodiazepines like phenazolam.
In 2025, phenazolam has been identified in eleven blood specimens (antemortem and postmortem) and 33 drug materials that were tested at our laboratory. The blood specimens originated from across the United States, as well as the United Kingdom. The majority of postmortem cases were males with ages from 18 to 31 years. Phenazolam has been detected alone and alongside other drugs including other benzodiazepines, opioids, stimulants, and hallucinogens. NPS detected alongside phenazolam included nitazene analogues, orphine analogues, medetomidine, and bromazolam, among others.
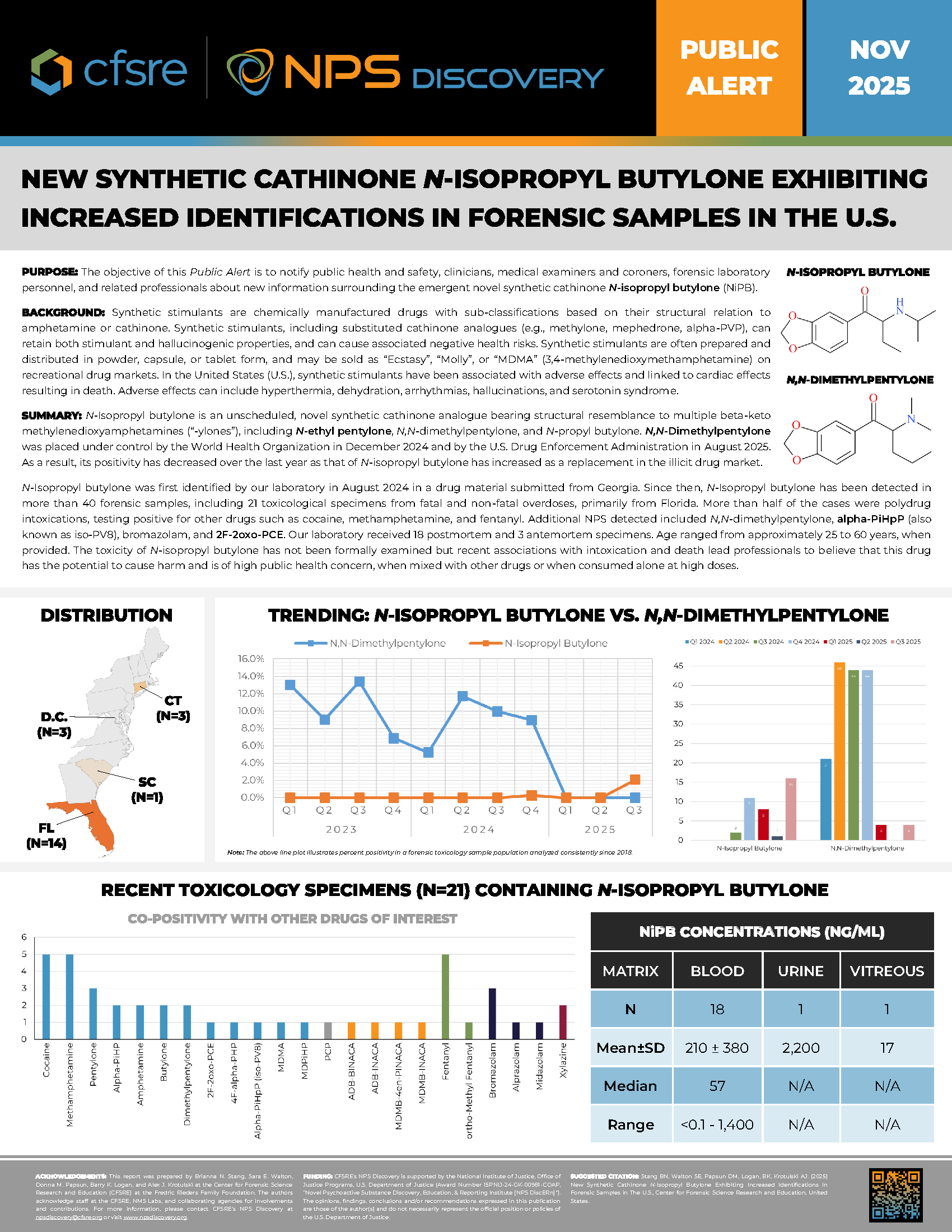
N-Isopropyl butylone is an unscheduled, novel synthetic cathinone analogue bearing structural resemblance to multiple beta-keto methylenedioxyamphetamines (“-ylones”), including N-ethyl pentylone, N,N-dimethylpentylone, and N-propyl butylone. N,N-Dimethylpentylone was placed under control by the World Health Organization in December 2024 and by the U.S. Drug Enforcement Administration in August 2025. As a result, its positivity has decreased over the last year as that of N-isopropyl butylone has increased as a replacement in the illicit drug market.
N-Isopropyl butylone was first identified by our laboratory in August 2024 in a drug material submitted from Georgia. Since then, N-Isopropyl butylone has been detected in more than 40 forensic samples, including 21 toxicological specimens from fatal and non-fatal overdoses, primarily from Florida. More than half of the cases were polydrug intoxications, testing positive for other drugs such as cocaine, methamphetamine, and fentanyl. Additional NPS detected included N,N-dimethylpentylone, alpha-PiHpP (also known as iso-PV8), bromazolam, and 2F-2oxo-PCE. Our laboratory received 18 postmortem and 3 antemortem specimens. Age ranged from approximately 25 to 60 years, when provided. The toxicity of N-isopropyl butylone has not been formally examined but recent associations with intoxication and death lead professionals to believe that this drug has the potential to cause harm and is of high public health concern, when mixed with other drugs or when consumed alone at high doses.
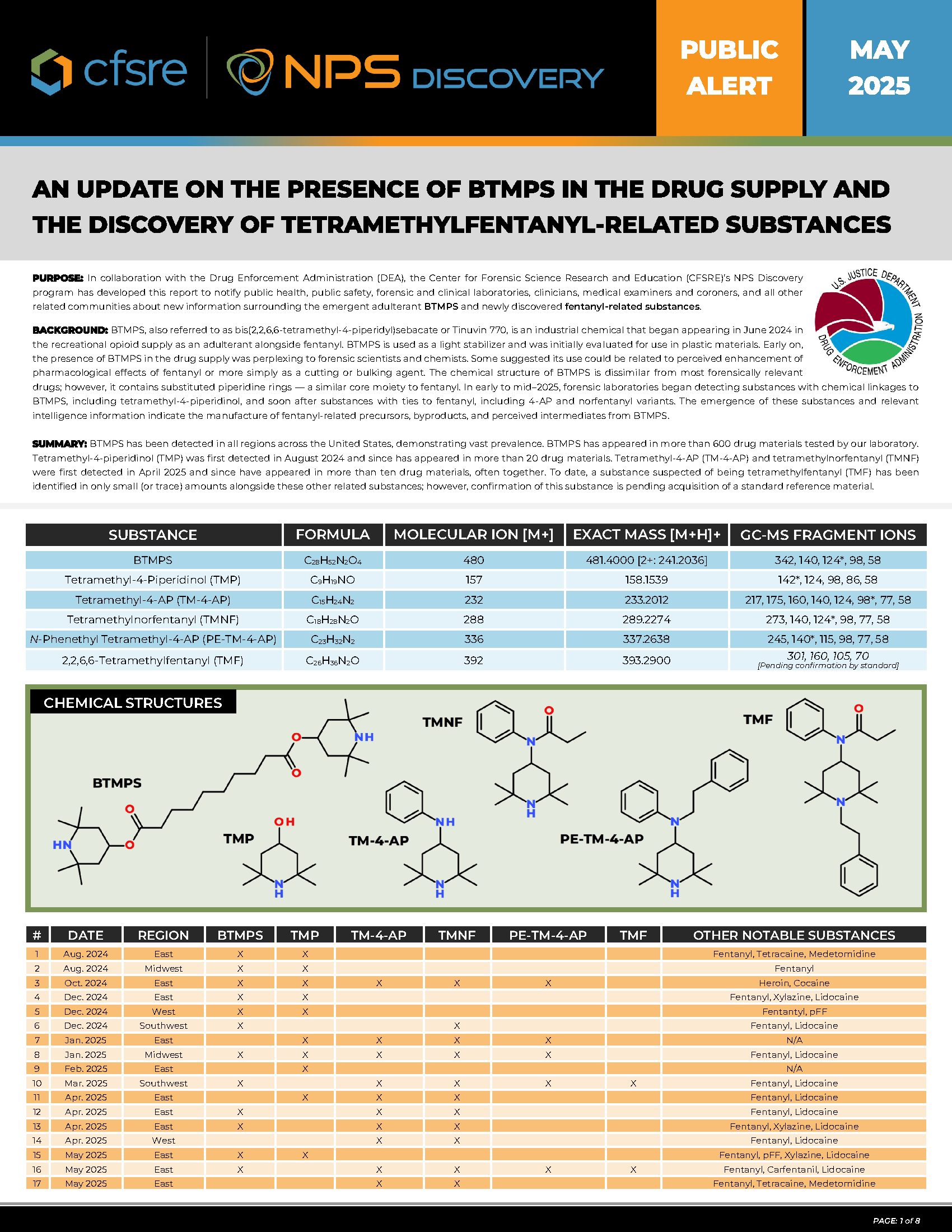
Purpose: In collaboration with the Drug Enforcement Administration (DEA), the Center for Forensic Science Research and Education (CFSRE)’s NPS Discovery program has developed this report to notify public health, public safety, forensic and clinical laboratories, clinicians, medical examiners and coroners, and all other related communities about new information surrounding the emergent adulterant BTMPS and newly discovered fentanyl-related substances.
Summary: BTMPS has been detected in all regions across the United States, demonstrating vast prevalence. BTMPS has appeared in more than 600 drug materials tested by our laboratory. Tetramethyl-4-piperidinol (TMP) was first detected in August 2024 and since has appeared in more than 20 drug materials. Tetramethyl-4-AP (TM-4-AP) and tetramethylnorfentanyl (TMNF) were first detected in April 2025 and since have appeared in more than ten drug materials, often together. To date, a substance suspected of being tetramethylfentanyl (TMF) has been identified in only small (or trace) amounts alongside these other related substances; however, confirmation of this substance is pending acquisition of standard reference material.
Public health and public safety officials worldwide should be aware of an emerging threat of the Benzimidazole (Nitazene) class of opioids, which are causing increased mortality (death) and morbidity. Considered several times more potent than the fentanyl class of opioids (phenylpiperidines), these compounds can make an existing opioid epidemic much worse or introduce an epidemic to unsuspecting countries and regions.
In 2023, nitazene tablets destined for Florida, Connecticut, and Brazil containing an average of 29 mg of metonitazene across multiple shipments were seized in the U.S. from international express mail. This amount is equivalent to 290 mg of fentanyl in a single tablet (or 145 times the DEA’s estimated fatal dose of fentanyl), which would be highly lethal.
DOWNLOAD THE ALERT
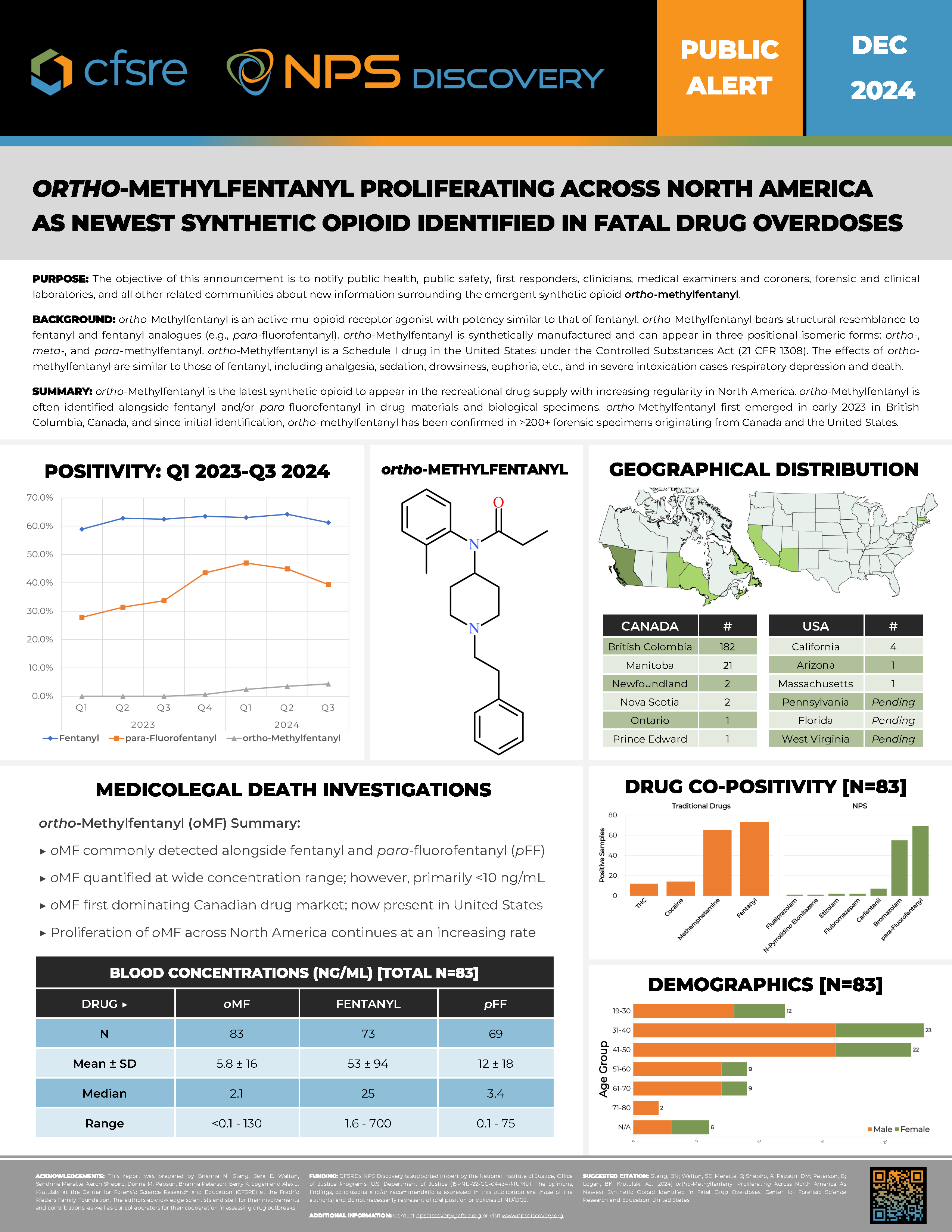
ortho-Methylfentanyl is an active mu-opioid receptor agonist with potency similar to that of fentanyl. ortho-Methylfentanyl bears structural resemblance to fentanyl and fentanyl analogues (e.g., para-fluorofentanyl). ortho-Methylfentanyl is synthetically manufactured and can appear in three positional isomeric forms: ortho-, meta-, and para-methylfentanyl. ortho-Methylfentanyl is a Schedule I drug in the United States under the Controlled Substances Act (21 CFR 1308). The effects of ortho-methylfentanyl are similar to those of fentanyl, including analgesia, sedation, drowsiness, euphoria, etc., and in severe intoxication cases respiratory depression and death.
ortho-Methylfentanyl is the latest synthetic opioid to appear in the recreational drug supply with increasing regularity in North America. ortho-Methylfentanyl is often identified alongside fentanyl and/or para-fluorofentanyl in drug materials and biological specimens. ortho-Methylfentanyl first emerged in early 2023 in British Columbia, Canada, and since initial identification, ortho-methylfentanyl has been confirmed in >200+ forensic specimens originating from Canada and the United States.
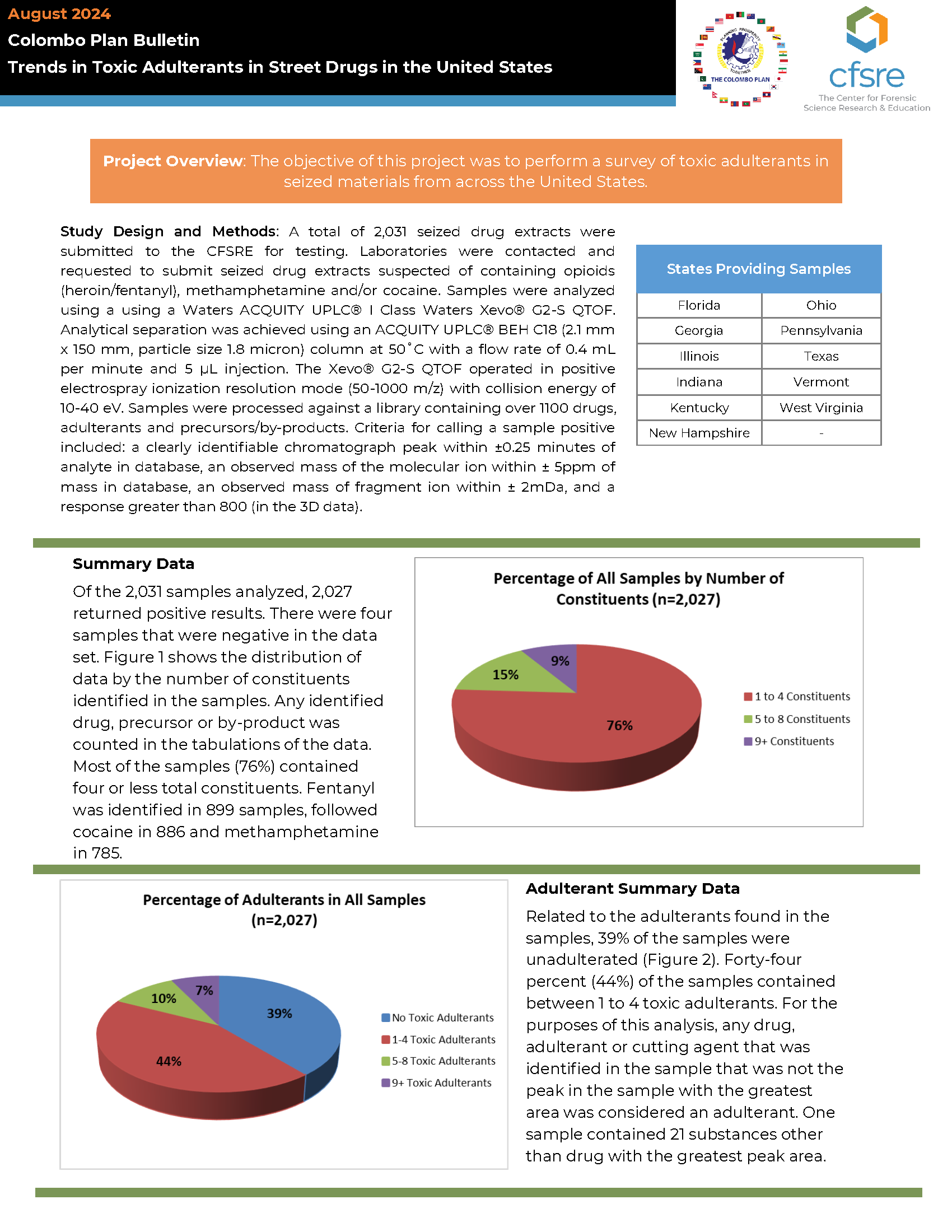
Project Overview: The objective of this project was to perform a survey of toxic adulterants in seized materials from across the United States.
Study Design and Methods: A total of 2,031 seized drug extracts were submitted to the CFSRE for testing. Laboratories were contacted and requested to submit seized drug extracts suspected of containing opioids (heroin/fentanyl), methamphetamine and/or cocaine. Samples were analyzed using a using a Waters ACQUITY UPLC® I Class Waters Xevo® G2-S QTOF. Analytical separation was achieved using an ACQUITY UPLC® BEH C18 (2.1 mm x 150 mm, particle size 1.8 micron) column at 50˚C with a flow rate of 0.4 mL per minute and 5 µL injection. The Xevo® G2-S QTOF operated in positive electrospray ionization resolution mode (50-1000 m/z) with collision energy of 10-40 eV. Samples were processed against a library containing over 1100 drugs, adulterants and precursors/by-products. Criteria for calling a sample positive included: a clearly identifiable chromatograph peak within ±0.25 minutes of analyte in database, an observed mass of the molecular ion within ± 5ppm of mass in database, an observed mass of fragment ion within ± 2mDa, and a response greater than 800 (in the 3D data).
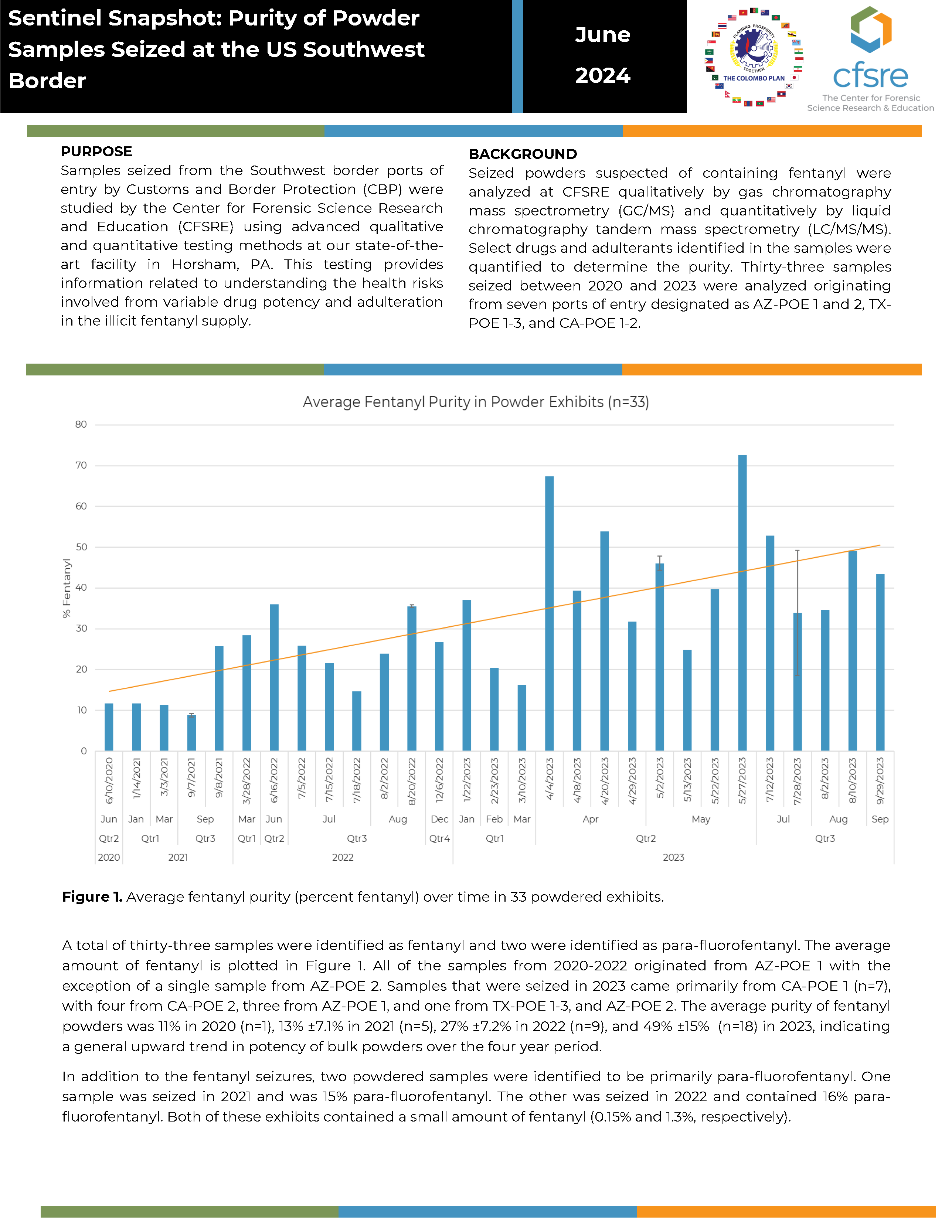
PURPOSE: Samples seized from the Southwest border ports of entry by Customs and Border Protection (CBP) were studied by the Center for Forensic Science Research and Education (CFSRE) using advanced qualitative and quantitative testing methods at our state-of-the-art facility in Horsham, PA. This testing provides information related to understanding the health risks involved from variable drug potency and adulteration in the illicit fentanyl supply.
BACKGROUND: Seized powders suspected of containing fentanyl were analyzed at CFSRE qualitatively by gas chromatography mass spectrometry (GC/MS) and quantitatively by liquid chromatography tandem mass spectrometry (LC/MS/MS). Select drugs and adulterants identified in the samples were quantified to determine the purity. Thirty-three samples seized between 2020 and 2023 were analyzed originating from seven ports of entry designated as AZ-POE 1 and 2, TX-POE 1-3, and CA-POE 1-2.
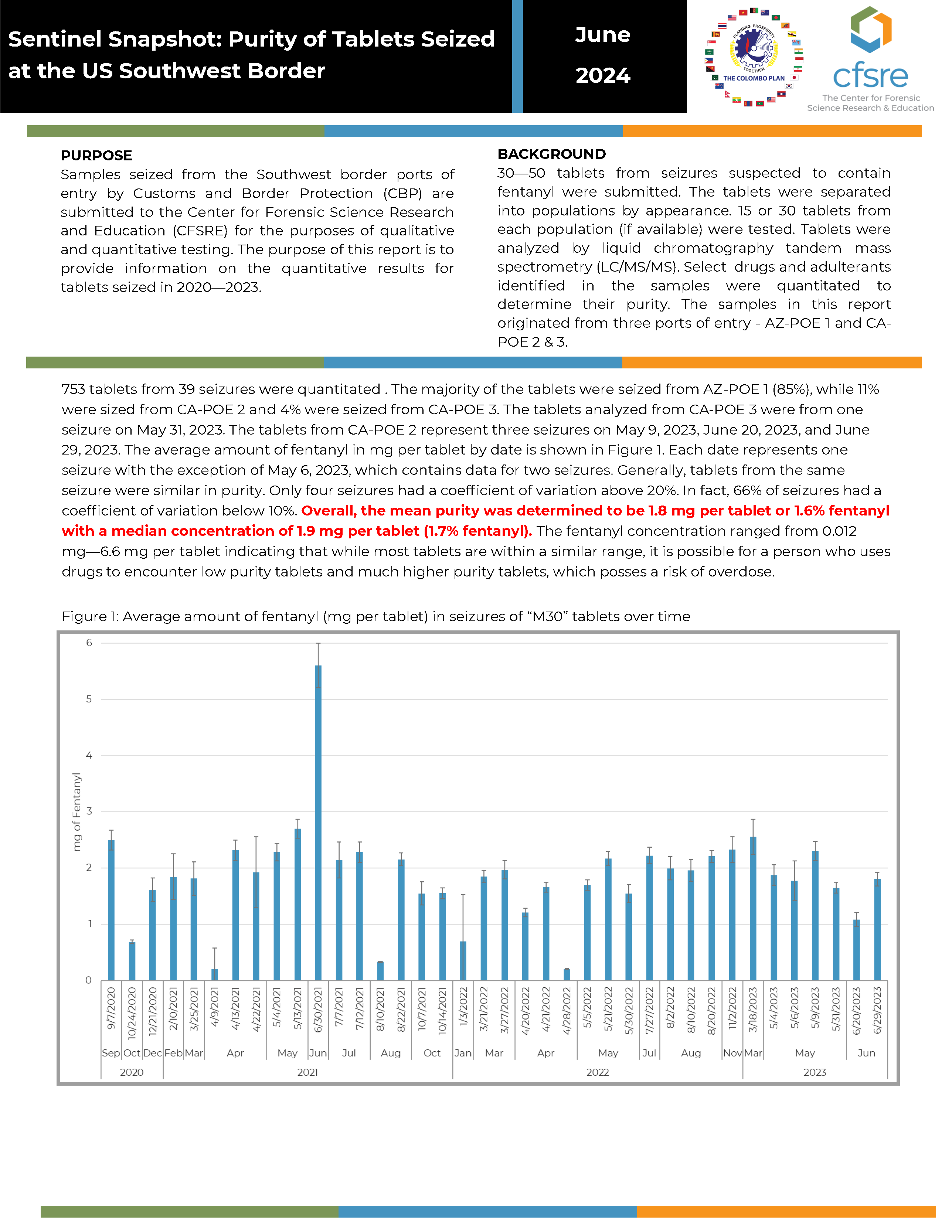
PURPOSE: Samples seized from the Southwest border ports of entry by Customs and Border Protection (CBP) are submitted to the Center for Forensic Science Research and Education (CFSRE) for the purposes of qualitative and quantitative testing. The purpose of this report is to provide information on the quantitative results for tablets seized in 2020—2023.
BACKGROUND: 30—50 tablets from seizures suspected to contain fentanyl were submitted. The tablets were separated into populations by appearance. 15 or 30 tablets from each population (if available) were tested. Tablets were analyzed by liquid chromatography tandem mass spectrometry (LC/MS/MS). Select drugs and adulterants identified in the samples were quantitated to determine their purity. The samples in this report originated from three ports of entry - AZ-POE 1 and CA-POE 2 & 3.