Rilmazafone
The following information was compiled in November 2023 and is subject to change as new research is conducted and as new information becomes available:
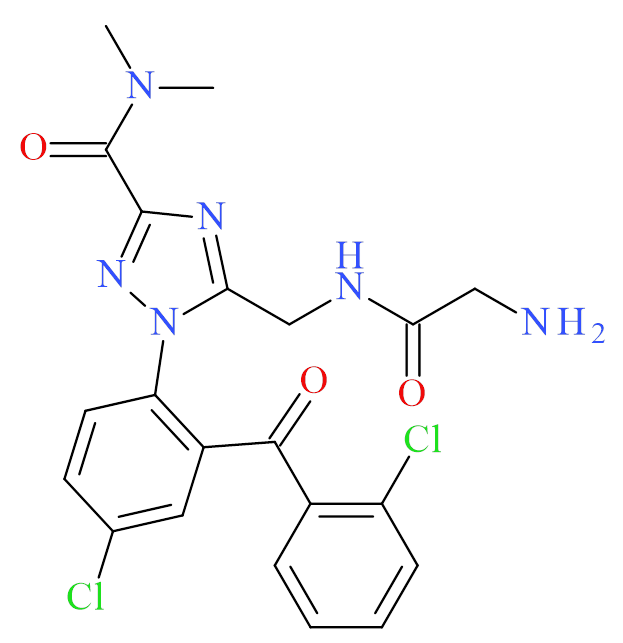
Description: Rilmazafone is a benzodiazepine-type drug that has recently emerged in the recreational drug supply. As a prodrug, rilmazafone is converted in the body to the benzodiazepine rilmazolam (below), which is the analyte expected to appear in toxicology specimens; however, standard reference material for rilmazolam is not currently available. Rilmazafone and its metabolites were reported in two fatal intoxications involving the drug in Europe.1
Sample Source: PA Groundhogs (Pennsylvania)
Sample Appearance: Pills
Pharmacology: The pharmacology of rilmazafone is extensively published in the literature.2
Toxicology: As expected, rilmazafone has not been detected in toxicology cases at the CFSRE.
Drug Materials: Rilmazafone has been detected in two drug materials at the CFSRE.
Demographics / Geographics: Drug materials originated from the state of Pennsylvania.
Legal Status: Rilmazafone is not explicitly scheduled in the United States.
- Class:
- Benzodiazepine
- Appearance:
- Pill
- Formula:
- C21H20Cl2N6O3
- MW:
- 475.3
- [M+]:
- 474
- [M+H]+:
- 475.1047
- IUPAC:
- 5-[[(2-aminoacetyl)amino]methyl]-1-[4-chloro-2-(2-chlorobenzoyl)phenyl]-N,N-dimethyl-1,2,4-triazole-3-carboxamide
- Report Date:
- November 29, 2023